FSHD therapeutics
Treatment for FSHD
In FSHD, the DUX4 protein needs to be removed or destroyed, or its pathogenic activity needs to be blocked. So, FSHD is amenable to numerous therapeutic technologies.
TAKE A DEEP DIVE INTO THERAPEUTIC APPROACHES FOR FSHD BY DIGGING INTO THE INFORMATION BELOW. AND BE SURE TO VIEW THE CRISPR INHIBITION VIDEO FOR AN OVERVIEW OF HOW IT IS IDEALLY SUITED FOR FSHD TO CORRECT MUSCLE CELLS WITHOUT MAKING PERMANENT CHANGES TO DNA.

CRISPR inhibition system for FSHD to correct muscle cells without making permanent changes to DNA.
Major breakthrough in gene therapy for neuromuscular diseases
Dive deep ...
Learn about treatment for FSHD in the area below. Click the red accordion bars to open/close them.
- See the phases and timelines that make up the path to a therapy once an IND (Investigational New Drug) filing is submitted to the FDA.
Losmapimod is an FDA-approved p38 inhibitor in clinical trial with Fulcrum Therapeutics.
Gene therapy approaches use the delivery of exogenous genetic material (typically DNA) to change the course of a disease.
Therapeutic CRISPR technology covers a wide range of approaches that utilize the highly specific sequence targeting capacity of the bacterial CRISPR/Cas systems to cut, edit or modulate the activity of DNA and RNA in human cells.
- Learn about CRISPR technology applicable to FSHD.
- Scalpel or Straitjacket: CRISPR/Cas9 Approaches for Muscular Dystrophies.
- See the original CRISPR-inhibition work by Dr. Charis Himeda, PhD.
- Read about the next step toward taking CRISPR inhibition to the clinic.
- Gene Editing Targeting the DUX4 Polyadenylation Signal: A Therapy for FSHD?
RNAi (RNA interference) is a natural biological mechanism evolved to maintain an organism’s genome integrity.
ASO therapeutic approaches can target the processing of specific RNAs, block their translation into protein, or target them for degradation. Therapeutic ASO approaches are already in clinic (and many more are in the pipeline) for a variety of diseases.
Myostatin inhibition is an approach to treat muscle diseases by “tricking” your body into making more muscle mass.
FSHD model organisms
The drive to develop treatments and a cure for FSHD requires the ethical use of animals and FSHD-like models in research. Animal research, including using mice, zebrafish, pigs, frogs, and nonhuman primates (required for toxicology) is vital to developing and getting an experimental FSHD therapy safely to the clinic and ultimately to patients.
We understand that this can be a sensitive issue. Unfortunately, there is a lot of misinformation out there. We will not find a cure for FSHD without the limited use of appropriate animal models and systems.
All animal research in the USA is governed by the U.S. Department of Agriculture and Animal Welfare Act, which sets high standards of care for research animals. The U.S. Public Health Service (PHS) Act requires that all institutions receiving federal funding from the National Institutes of Health (NIH), the Food and Drug Administration (FDA), or the Centers for Disease Control and Prevention (CDC) adhere to the standards set forth in the Guide for the Care and Use of Laboratory Animals.
Animal research is overseen locally at each PHS-funded institution by an Institutional Animal Care and Use Committee (IACUC). IACUCs include a veterinarian and an unaffiliated community representative. IACUCs require researchers to justify the need for animals and to use the lowest possible numbers to answer a specific question. IACUCs can reject inadequate proposals.
Scientists are mandated to adhere to the 3Rs: Reduction, Replacement, and Refinement to reduce the overall number of animals used in a study. Animal research must be conducted ethically at all times, and only when alternatives to obtaining the required information in a whole living system are not available.
Many muscular dystrophies and neuromuscular diseases use natural or induced animal models of the disease caused by mutations in the endogenous animal (mouse or dog) genome giving rise to the disease in that model animal system. Alternatively, the endogenous disease-causing genes can be studied for their normal and pathogenic functions using mice or dogs since their genes are very similar to the human gene.
However, while the DUX family of genes is found in all mammals, the DUX4 gene that causes FSHD is specific to old world primates (i.e. humans, gorillas, chimpanzees, etc.), none of which are used in biomedical research anymore and will not be used in FSHD research.
Thus, in order to generate FSHD in a model organism, one must express the human DUX4 gene in the desired system. Fortunately, since FSHD is a dominant gain-of-function disease, there are many techniques available to do this; however, it is technically challenging since DUX4 is very toxic. On this page, see: Approaches for studying DUX4 expression and preclinical testing FSHD therapeutics in animal models.


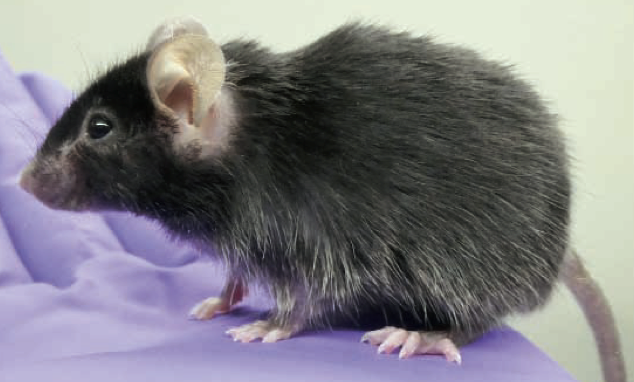
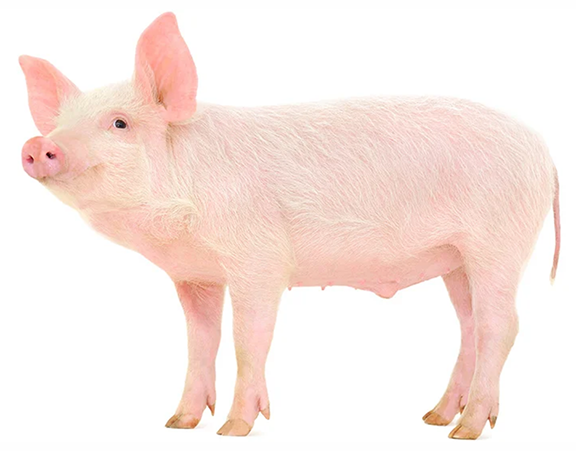
FSHD research has used small model organisms — Drosophila melanogaster (fruit fly), Xenopus laevis (African clawed frog), Danio rerio (zebrafish) and Mus musculus (mouse) — to better understand FSHD pathogenic mechanisms, model disease, and perform preclinical testing of potential FSHD therapeutics. The Jones Lab is collaborating with industry to make large animal models of FSHD using Göttingen minipigs (Sus scrofa). The FLExDUX4 FSHD-like minipigs became available in November 2023. Contact Precigen-Exemplar for more information. The FSHD1 and FSHD2 models are still under development.
TAKE A DEEP DIVE INTO FSHD MODEL ORGANISMS BY DIGGING INTO THE INFORMATION BELOW. AND BE SURE TO VIEW THE VIDEOS ABOUT THE INDUCIBLE DUX4 MOUSE MODEL FOR FSHD AND FSHD MINIPIGS MODEL.
Approaches for studying DUX4 expression and preclinical testing FSHD therapeutics in animal models
Inject DUX4 mRNA into developing embryos
— Xenopus laevis (Wuebbles et al. 2010. Int. J. Exp. Pathol 3:388-400.)
— Zebrafish (Mitsuhashi et al. 2013. Human Molecular Genetics 22:568-77.)
Inject virus expressing DUX4 into muscle (Wallace et al. 2011. Ann Neurology 69:540-52.)
Electroporate DUX4 plasmid into muscle (Derenne et al. 2020. Scientific Reports 10:11301.)
Make transgenic mouse with the D4Z4 region (Krom et al. 2013. PLoS Genetics 9:e1003415.)
Make transgenic animals expressing DUX4
— Fly (Jones et al. 2016. PLoS One 11:e0150938.)
— Mouse
(Bosnakovski et al. 2017 Nature Communications 8:550.)
(Jones et al. 2018. PLoS One 13:e0192657.)
(Giesige et al. 2018. JCI Insight 3:e123538.)
— Zebrafish (Pakula et al. 2019. Human Molecular Genetics 28:320-331.)
Make human xenografts of FSHD muscle in mice (Mueller et al. 2019. Exp Neurol 320:113011.)
More information on the necessity and benefit of using animals in biomedical research:
Inducible DUX4 mouse model for FSHD
FSHD minipigs: a tool to help move FSHD therapies to clinic
Dive deep ...
Learn about FSHD model organisms in the area below. Click the red accordion bars to open/close them.
The drive to develop treatments and a cure for FSHD requires the ethical use of animals and FSHD-like models in research.
- Read an overview of FSHD model organisms.
For an excellent review of FSHD models, see: DeSimone et al., “Cellular and animal models for facioscapulohumeral muscular dystrophy” (2020) Disease Models and Mechanisms 13(10):dmm046904.
FSHD-like model mice are critical tools for FSHD research. Learn about the ways researchers express human DUX4 in these FSHD mouse models:
- Mouse model overview.
- FLExDUX4 FSHD-like mouse.
To learn more about the science behind the FLEx DUX4 FSHD-like mice, see the original publications:
— A cre-inducible DUX4 transgenic mouse model for investigating facioscapulohumeral muscular dystrophy.
— Transgenic mice expressing tunable levels of DUX4 develop characteristic facioscapulohumeral muscular dystrophy-like pathophysiology ranging in severity. - Human FSHD xenograft mice.
To learn more about the science behind the human FSHD xenograft mice, see the original publication:
— Muscle xenografts reproduce key molecular features of facioscapulohumeral muscular dystrophy.
The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use guidelines require the use of two species in non-clinical studies for pharmaceuticals: one rodent and one non-rodent species.
With its many anatomical, physiological and functional similarities to humans, the minipig is an excellent alternative to dogs or non-human primates for clinical safety testing, drug dosing and human disease modeling.
Ethical animal research, including using mice, zebrafish, pigs and frogs, is vital to developing and getting an experimental FSHD therapy safely to the clinic and ultimately to patients. Read about the research using these FSHD model organisms:
Ultimately, FSHD is uniquely human and, whenever possible, maintaining the human context is the best system. Human cellular systems include muscle biopsy, muscle biopsy-derived muscle cells, immortalized myoblasts, fibroblasts, lymphoblastoid cells, and induced pluripotent stem cells (iPSCs).
Participate in FSHD research
RESEARCH PROJECT TO IMPROVE FSHD DIAGNOSTICS AND TO BETTER UNDERSTAND THE GENETICS AND EPIGENETICS OF FSHD
The Peter and Takako Jones Lab, located at the University of Nevada, Reno School of Medicine (Reno, Nevada), is recruiting participants to further validate this more affordable and accessible testing procedure to become an accepted and approved FSHD diagnostic option. There is no cost to participate or to have your research results returned directly to you.
- Principal Investigator: Peter L. Jones, PhD.
- Sponsor: University of Nevada, Reno School of Medicine, USA.
GAIT ASSESSMENT FOR FSHD
Dr. Nicholas Murray, PhD, and Dr. Ryan Wuebbles, PhD, are recruiting participants for their Gait Assessment to study the natural history of the gait cycle, or walking patterns, of individuals with facioscapulohumeral muscular dystrophy (FSHD) and to analyze how this gait cycle may be impacted by the disease.
There is no cost to participate, and this assessment is done by you in your home or personal setting, using a mobile phone they will provide you that is equipped with the gait analyzer app.
To participate or for more information, email Dr. Nicholas Murray, PhD, Director of the Neuromechanics Laboratory at the University of Nevada, Reno.
Participate in FSHD clinical trials
MOTOR OUTCOMES TO VALIDATE EVALUATIONS IN FSHD (MOVE FSHD)
(ClinicalTrials.gov Identifier NCT04635891)
The primary goal of this proposal is to collect motor and functional outcomes specific to FSHD over time. By collecting measures specific to FSHD, this will help ensure the best level of clinical care is being provided. Also, the hope is to speed up drug development by gaining a better understanding of how having FSHD impacts motor function and other health outcomes (i.e. breathing, wheelchair use, etc.) and how big a change in motor function would be clinically meaningful to those with FSHD.
More about MOVE FSHD and participating locations
Responsible party
University of Kansas Medical Center
Contact: Michaela Walker | 913-945-9920 | mwalker20@kumc.edu
Contact: Kiley Higgs | 913-945-9922 | ksims2@kumc.edu
CLINICAL TRIAL READINESS TO SOLVE BARRIERS TO DRUG DEVELOPMENT IN FSHD
(ClinicalTrials.gov Identifier: NCT03458832)
The primary cause of facioscapulohumeral muscular dystrophy (FSHD), a common adult-onset dystrophy, was recently discovered identifying targets for therapy. As multiple drug companies pursue treatments for FSHD, there is an urgent need to define the clinical trial strategies which will hasten drug development, including creating disease-relevant outcome measures and optimizing inclusion criteria. This proposal will develop two new outcome measures and optimize eligibility criteria by testing 160 patients in 7 sites over a period of 18 months.
More about the clinical trial readiness to solve barriers to drug development in FSHD
Responsible party
University of Kansas Medical Center
Contact: Kiley Higgs | 913-945-9922 | ksims2@kumc.edu
Find a clinical trial
Patient registries
According to National Institutes of Health, a patient registry is a collection of information about individuals, usually focused around a specific diagnosis or condition. Individuals provide information about themselves to these registries on a voluntary basis. Registries can be sponsored by a government agency, nonprofit organization, health care facility, or private company.
- National Registry for Myotonic Dystrophy (DM) & Facioscapulohumeral Dystrophy (FSHD) (University of Rochester Medical Center, Rochester, New York)
- UK FSHD Registry at John Walton Muscular Dystrophy Research Centre
Chiara Marini Bettolo
Institute of Translational and Clinical Research
Faculty of Medical Sciences
Newcastle University
Central Parkway
Newcastle upon Tyne
NE1 3BZ
chiara.Marini-Bettolo@newcastle.ac.uk - Patient registries around the world