About FSHD
The lowdown on FSHD
FSHD is indeed complicated and often confusing. It is highly variable in how and when it affects individuals. So, let’s have a brief overview of some FSHD facts and make sure we are all on the same page. Once acquainted with FSHD, then perhaps you can decide if you want to learn more about your own FSHD, or potential FSHD, by participating in FSHD research testing.
WHAT IS FSHD?
FSHD refers to facioscapulohumeral muscular dystrophy; however, the disease is also known in many parts of the world by its original name, Landouzy-Dejerine muscular dystrophy (or Landouzy Derjerine Syndrome), named after the two 19th-century French physicians who first published case histories of what would become known as FSHD patients and documenting them as having a distinct class of muscle disease (“De la myopathie atrophique progressive”, 1885, Rev Med Franc 5:81-117 and 253-366).
FSHD, which includes FSHD1 and FSHD2, is one of the nine classes of muscular dystrophy: Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), Limb-Girdle muscular dystrophy (LGMD1A-H and LGMD2A-Q), Myotonic dystrophy (DM1, DM2), Congenital muscular dystrophy (CMD), Emery-Dreifuss muscular dystrophy (EDMD1-7), and Oculopharyngeal muscular dystrophy (OPMD), and Distal muscular dystrophy (DD).
There are two classes of FSHD, FSHD1 (OMIM #158900) and FSHD2 (OMIM #158901), that are clinically indistinguishable and share the same fundamental pathogenic mechanisms, but have different genetics. Roughly 95% of people with FSHD are FSHD1.
FSHD1 is caused by genetic mutations at chromosome 4q35 that leads to a deletion within the D4Z4 array and aberrant expression of the DUX4 gene in skeletal muscles.
FSHD2 is caused by genetic mutations in genes that regulate DUX4 expression from the chromosome 4q35 D4Z4 array. You will find more about the genetics and epigenetics of FSHD elsewhere on this site.
Aberrant expression of the DUX4 gene in skeletal muscles leads to FSHD pathophysiology in both forms of FSHD.
For a more detailed discussion of FSHD, see this excellent eBook review.
WATCH THE FOLLOWING VIDEOS FOR OVERVIEWS OF FSHD. THEN, TAKE A DEEPER DIVE INTO WHAT FSHD IS BY DIGGING INTO THE INFORMATION BELOW THE VIDEOS AND VISITING OTHER PAGES ON THIS SITE.

What is FSHD?
FSHD1 & FSHD2 differences
What is FSHD1+2?
Dive deep ...
Learn more about FSHD in the area below. Click the red accordion bars to open/close them.
Slowly progressive muscle weakness involving the face, scapular stabilizers, upper arms, hip girdle, abdomen and lower legs, including the dorsiflexors of the foot.
FSHD can affect males and females of all ages. Symptoms typically appear in the second decade of life for males and the third for females, but age of onset is highly variable. There is a severe infantile form that presents between birth and age 10 and conversely, many genetically FSHD individuals may remain asymptomatic for most or all of their life.
Facial weakness is typically the first sign of FSHD, although it is often reported retrospectively, and includes difficulty whistling and drinking through a straw, sleeping with eyes partially open, and inability to purse one’s lips. Symptoms are more noticeable in the lower facial muscles than the upper facial muscle.
However, scapular winging, or upward movement of the scapula when flexing or abducting the arms, and accompanying weakness in the shoulder blades are often the first noticeable signs of FSHD. This weakness is very often asymmetric and readily visible from the back. Typically for FSHD, it may become difficult and eventually impossible to lift ones arms over the head, and this is a common self-reported feature that sends people to a neurologist because something is clearly wrong.
While one’s forearms are typically spared, the biceps and triceps often show atrophy and this is commonly asymmetric, with one arm being more affected than the other.
In fact, asymmetry of weakness involving many of the affected muscles is characteristic for FSHD.
Foot drop is common in FSHD. The muscles that raise the front of the foot become weak, and the foot will stay pointed down when walking, causing tripping, especially on uneven surfaces, or catching on a curb.
FSHD patients often have a positive Beevor’s sign, which is the upward movement of the navel when flexing one’s neck to raise their head. In unaffected individuals, the navel stays put. It is indicative of weakness in the lower abdominal muscles.
Lordosis refers to an exaggerated curve in the lower region of the spine due to weakness in the abdominal muscles. Hyperlordosis is caused by additional weakness of the pelvic extensor and paraspinal muscles in the back. These can be controlled to some degree by use of bracing. Potentially corrective surgery has been reported but is not common.
Chronic pain has been reported in as high as 88.6% of FSHD-affected people, with >30% characterizing it as severe. The pain is most commonly localized to the shoulders and lower back.
- Morris et al., “Chronic pain has a strong impact on quality of life in facioscapulohumeral muscular dystrophy” (2018) Muscle Nerve 57:380-7.
Some women have reported FSHD symptoms first appearing noticeable soon after pregnancy. However, a large study (85 participants) found that as a group, the majority of women with FSHD reported an unchanged rate of disease progression through periods of hormonal change suggesting that estrogen exposure does not have a clinically relevant effect (protective or harmful) on disease severity.
- Mul et al., “Lifetime endogenous estrogen exposure and disease severity in female patients with facioscapulohumeral muscular dystrophy” (2018) Neuromuscular Disorders 28:508-11.
Approximately 20% of FSHD-affected individuals will eventually require a wheelchair. This does correlate with the size of the FSHD1 deletion, with larger deletions (1-3 repeat units) being more likely to require wheelchair assistance and smaller deletions (7-10 repeat units) rarely, if ever, needing wheelchair assistance.
- Statland et al., “Milder phenotype in facioscapulohumeral dystrophy with 7-10 residual D4Z4 repeats” (2015) Neurology 85:2147-50.
FSHD symptoms typically present earlier and are overall more severe in males than in females with disease penetrance in males at age 30 significantly higher than in females at age 30; however, this is a trend through a large population and there are of course exceptions.
- Zatz et al., “The facioscapulohumeral muscular dystrophy (FSHD1) gene affects males more severely and more frequently than females” (1998) American Journal of Medical Genetics 77:155-61.
Another sign of FSHD, but not exclusive to FSHD, is that it often runs in families. FSHD has an autosomal dominant mode of inheritance. Thus, someone with FSHD1 has a 50% likelihood of passing their FSHD1 genetics to each child. In addition, due to the typical late onset or recognition of symptoms, many people are diagnosed with FSHD after having children. If one of your parents has been diagnosed with FSHD and you are showing signs, there is a good chance it is FSHD and not another neuromuscular condition. It should be noted that the presentation of FSHD symptoms can be highly variable within a family, even between siblings, and it is difficult to predict the course of disease based on other family members. In addition, depending upon the size of the FSHD1 deletion, there may be a number of asymptomatic family members who are genetically FSHD but show no clinical manifestation. If one of your parents has FSHD, you may want to get checked out regardless of symptoms; however, if you do decide to get tested, make sure you are mentally prepared for receiving the results, either way.
- Tonini et al., “Asymptomatic carriers and gender differences in facioscapulohumeral muscular dystrophy (FSHD)” (2004) Neuromuscular Disorders 14:33-8 and Jones et al., “Facioscapulohumeral muscular dystrophy family studies of DUX4 expression: …” (2012) Human Molecular Genetics 21:4419-30.
Overall, what makes FSHD so complicated, even in families with FSHD, is the great degree of variability in severity, age of onset, rate of progression, and affected muscles, all of which can vary between siblings and other first-degree relatives with FSHD.
FSHD clinical symptoms typically appear in the second or third decade of life for males and third or fourth decade for females; however, symptoms can appear from birth. Thus, early onset (or infantile) FSHD, is the subclass of patients with clinical symptoms appearing early in life. Individuals showing facial weakness before age 5 years and scapulohumeral weakness before age 10 years are considered early-onset.
- Brouwer et al. “Facioscapulohumeral muscular dystrophy in early childhood” (1994) Arch Neurology 51:387-94.
Early onset FSHD is typically associated with large deletions (1-3 D4Z4 repeat units) and is often more severe, progresses more rapidly, and is associated with extra-muscular manifestations of disease (discussed below) compared with the more common adult onset FSHD.
- Klinge et al. “Severe phenotype in infantile facioscapulohumeral muscular dystrophy” 2006. Neuromuscular Disorders 16:553-8 and Goselink et al. “Early onset as a marker for disease severity in facioscapulohumeral muscular dystrophy” (2019) Neurology 92:e378-85.
Alternatively, early onset and severe FSHD can be caused by FSHD1+2, whereby a typically non-severe FSHD1 deletion is coupled with an FSHD2 mutation (such as in the SMCHD1 gene) that together result in a much more severe disease than would be predicted for their size deletion. FSHD1 and FSHD2 are discussed further below and elsewhere on the site.
- Sacconi et al. “The FSHD2 gene SMCHD1 is a modifier of disease severity in families affected by FSHD1” (2013) American Journal of Human Genetics 93:744-51.
Respiratory insufficiency in FSHD
It is often stated in the clinical literature that FSHD does not affect the respiratory muscles and has no effect on one’s lifespan. However, this view is outdated as more evidence shows this is a critical clinical issue for more severe FSHD patients as disease progresses.
In fact, evaluation of systemic pulmonary function in patients complaining of sleep-related disorders identified impairment of expiratory muscles.
- Santos et al., “Respiratory muscle dysfunction in facioscapulohumeral muscular dystrophy” (2020) Sleep and Breathing 24:603-4.
A separate study found respiratory muscle weakness involving the diaphragm and expiratory abdominal muscles independent of reported sleep disorders and concluded that respiratory muscle weakness is an intrinsic feature of FSHD.
- Henke et al., “Respiratory muscle weakness in facioscapulohumeral muscular dystrophy” (2019) Muscle Nerve 60:679-86.
Pediatric FSHD patients were found to have altered respiratory volumes that correlated with clinical severity, again suggesting expiratory muscle weakness.
- Trucco et al., “Respiratory pattern in a FSHD pediatric population” (2016) Respiratory Medicine 119:78-80.
A common question is if wheelchair dependency in FSHD is a risk factor for developing respiratory insufficiency. Two recent studies of ambulant and wheelchair-restricted FSHD patients have been performed. The first study consisted of 81 FSHD patients, none of whom complained of respiratory dysfunction, and found that more than one-third of the wheelchair-dependent FSHD patients did in fact have respiratory insufficiency ranging from mild to severe. The wheelchair-dependent group with accompanying kyphosis showed the most restrictive respiratory function. Interestingly, in contrast with other studies, the ambulant group did not show any respiratory dysfunction.
- Wohlgemuth et al., “Respiratory function in facioscapulohumeral muscular dystrophy 1” (2017) Neuromuscular Disorders 27:526-30.
A second study of 94 FSHD patients, with 68 being ambulant and 26 being wheelchair dependent, found respiratory insufficiency in both groups, and correlating with clinical severity score with 38% (10/26) of the wheelchair dependent group showing severe respiratory involvement.
- Moreira et al., “Respiratory involvement in ambulant and non-ambulant patients with facioscapulohumeral muscular dystrophy” (2017) Journal of Neurology 264:1271-80.
FSHD patients who complain of excessive daytime sleepiness, morning headaches, and/or non-restorative sleep, may be suffering from several sleep-related breathing disorders associated with more severe manifestation of disease, including obstructive sleep apnea, nocturnal hypoventilation, or the combination of both. A recent sleep study resulted in the recommendation of night-time non-invasive ventilation for ~20% (6/31) of such patients, with improvement seen the first night of treatment. A separate, independent study with different inclusion criteria found >80% of FSHD patients showed altered respiration capacity and recommended non-invasive ventilation for 3% of enrolled patients.
- Runte et al., “Sleep-related breathing disorders in facioscapulohumeral dystrophy” (2019) Sleep and Breathing 23:899-906 and Santos et al., “Respiratory muscle dysfunction in facioscapulohumeral muscular dystrophy” (2020) Sleep and Breathing 24:603-4.
Overall, respiratory insufficiency is a major concern in FSHD and may in fact impact your health risk and lifespan if ignored. The above studies recommend that patients, particularly wheelchair-dependent individuals, should be evaluated periodically regardless of self-reported respiratory problems to determine if intervention would be beneficial.
Extra-muscular manifestations of FSHD
Severe cases of FSHD may have non-muscular manifestations, including retinal vasculopathy and high-frequency hearing loss.
- Gieron et al. (1985) Am J Medical Genetics 22:143-7; Taylor et al. (1982) Annals of Neurology 12:395-8; Padberg et al. (1995) Muscle Nerve 2:S73-80; Statland et al. (2013) Neurology 80:1247-50.
Retinal vasculopathy, or retinal vascular disease, consisting of peripheral retinal capillary abnormalities as visualized by fluorescein angiography, is a common feature of FSHD. However, the retinal vasculopathy does not typically affect one’s vision, with retinal detachment and vision loss happening only in rare cases (<1%) and is more commonly associated with the early-onset FSHD.
- Fitzsimons et al. (1987) “Retinal vascular abnormalities in facioscapulohumeral muscular dystrophy. A general association with genetic and therapeutic implications” Brain 110:631-48.
High-frequency hearing loss is part of FSHD for some patients and may result in more severe hearing loss. However, it is most common in those individuals with larger FSHD deletions (smaller D4Z4 arrays <20kb) and early onset of muscle weakness.
- Brouwer et al. “Hearing loss in facioscapulohumeral muscular dystrophy” (1991) Neurology 41:1878-81 and Lutz et al., “Clinical and genetic features of hearing loss in facioscapulohumeral muscular dystrophy” (2013) Neurology 81:1374-77.
For excellent reviews covering clinical FSHD, see:
- Leo Wang and Rabi Tawil, “Facioscapulohumeral Dystrophy” (2016) Current Neurology and Neuroscience Reports 16:66.
- Karlien Mul et al., “What’s in a name? The clinical features of facioscapulohumeral muscular dystrophy” (2016) Practical Neurology 16:201-7.
Patient-driven project covering FSHD-related characteristics
There are two genetic classes of FSHD, FSHD1 (OMIM #158900) and FSHD2 (OMIM #158901).
FSHD1 is caused by contractions within the chromosome 4q35 D4Z4 macrosatellite array.
- Wijmenga et al., “Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy” (1992) Nature Genetics 2:26-30 and van Deutekom et al., “FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit” (1993) Human Molecular Genetics 2:2037-42.
FSHD1 has an autosomal dominant mode of inheritance; thus, there is a 50% likelihood of passing the disease on to each child.
- Tyler et al., “Clinical manifestations and inheritance of facioscapulohumeral dystrophy in a large family” (1950) Ann Intern Med 32:640-60.
FSHD2 is caused by mutations in genes that regulate gene repression of the 4q35 D4Z4 macrosatellite array, including SMCHD1, DNMT3B, and LRIF1.
- Lemmers et al., “Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes FSHD2” (2012) Nature Genetics 44:1370-4, van den Boogaard et al. “Mutations in DNMT3B modify epigenetic repression of the D4Z4 repeat and the penetrance of FSHD” (2016) Am J Human Genetics 98:1020-9, and Hamanaka et al. “Homozygous nonsense variant in LRIF1 associated with facioscapulohumeral muscular dystrophy” (2020) Neurology 94:e2441-7.
For excellent reviews of FSHD genetics and epigenetics, see:
- Anna Greco, Remko Goossens, Baziel van Engelen, and Silvere M. van der Maarel, “Consequences of epigenetic derepression in facioscapulohumeral muscular dystrophy” (2020) Clinical Genetics 97:799-814.
- Charis L. Himeda and Peter L. Jones, “The Genetics and Epigenetics of Facioscapulohumeral Muscular Dystrophy” (2019) Annual Reviews of Genomics and Human Genetics 20:265-91.
- Nicholas Johnson and Jeffery Statland, “FSHD1 or FSHD2: That is the question. The answer: It’s all just FSHD” editorial (2019) Neurology 92:881-882.
- Lucia Daxinger, Stephen J Tapscott, and Silvere M van der Maarel. “Genetic and epigenetic contributors to FSHD” (2015) Current Opinions in Genetics and Development 33:56-61.
- Rabi Tawil, Silvere M van der Maarel and Stephen J Tapscott, “Facioscapulohumeral dystrophy: the path to consensus on pathophysiology” (2014) Skeletal Muscle 4:12.
- Richards et al., “Facioscapulohumeral muscular dystrophy (FSHD): an enigma unraveled?” (2012) Human Genetics 131:325-40.
FSHD is caused by the aberrant expression of the DUX4 gene from within an epigenetically dysregulated chromosome 4q35 D4Z4 array leading to a pathogenic cascade of events ultimately resulting in FSHD pathophysiology.
The DUX4 gene is encoded in each D4Z4 repeat unit. The D4Z4 had been thought to be noncoding DNA. While the exact sequence reported here is not quite right, likely due to a sequencing error, it is fundamentally correct that each D4Z4 repeat encodes a paired homeobox domain transcription factor. Thus, this represented a new FSHD disease candidate gene in the FSHD region; however, it was not widely accepted to be involved in FSHD until 2010. Turns out this study had it right.
- Gabriels et al., Nucleotide sequence of the partially deleted D4Z4 locus in a patient with FSHD identifies a putative gene within each 3.3kb element” (1999) Gene 236:25-32.
Expression of the DUX4 gene can cause apoptotic cell death when expressed in somatic cells. This is consistent with DUX4 protein having an adverse effect on muscle cells and a gain-of-function disease mechanism in FSHD. This was the first study to show that the D4Z4 did in fact encode a functional protein, DUX4, and it was bad for cells.
- Kowaljow et al., “The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein” (2007) Neuromuscular Disorders 17:611-23.
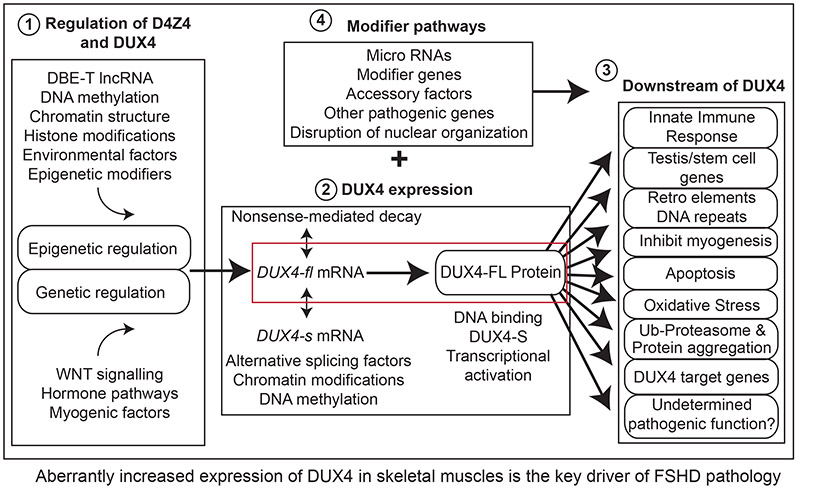
A pathogenic increase in expression of the DUX4 gene from the chromosome 4q35 D4Z4 macrosatellite array in skeletal muscle causes both forms of FSHD. Thus, FSHD1 and FSHD2 are both genetically linked to the chromosome 4q35 D4Z4 array and DUX4 expression. One cannot be FSHD1 or FSHD2 if they do not have an FSHD permissive chromosome 4A, and here it is shown that being FSHD-permissive is directly connected to being able to express the DUX4 gene due to polyadenylation of the DUX4 mRNA.
- Lemmers et al., “A unifying genetic model for facioscapulohumeral muscular dystrophy” (2010) Science 329:1650-3 and Snider et al., “Facioscapulohumeral muscular dystrophy: Incomplete suppression of a retrotransposed gene” (2010) PLoS Genetics 6:e1001181.
DUX4 expression in FSHD is not black and white, on and off; instead it is shades of gray. While the original reports showed FSHD cells express DUX4 and healthy cells did not express DUX4, many labs could not repeat this clean result. Here, we learned that some healthy muscle cells do in fact express DUX4 and many FSHD muscle cells do not. However overall, in muscle cell cultures and muscle biopsies, FSHD-derived muscle cells express significantly more DUX4 than healthy muscle cells; it is the levels of DUX4 that are different. This is significant therapeutically, too, in that it proves that healthy muscle can tolerate some degree of DUX4 expression, and FSHD drugs just need to dial back, not eliminate, DUX4 expression.
- Jones et al., “Facioscapulohumeral muscular dystrophy family studies of DUX4 expression: evidence for disease modifiers and a quantitative model of pathogenesis” (2012) Human Molecular Genetics 21:4419-30.
Importantly, the gene expression profile induced by the DUX4 gene when expressed in muscle cells, the “DUX4 signature”, is readily apparent as the main aberrant transcriptional signature in FSHD muscle compared with healthy muscle. In addition, a secondary signature suggests an immune cell infiltration in the FSHD muscle. This is very important as is strongly supports that DUX4 expression alone is causal for FSHD.
- Yao et al., “DUXx4-induced gene expression is the major molecular signature in FSHD skeletal muscle” (2014) Human Molecular Genetics 23:5342-52.
Expression of DUX4 in the skeletal muscles of mice recapitulates many features of FSHD pathophysiology. This was very important because the first mouse model of FSHD produced by the van der Maarel lab, the D4Z4-2.5 mouse, did not get an FSHD-like phenotype. It was in fact quite healthy. This led fuel to the fire for those who opposed the DUX4 model for FSHD. Three groups generated slightly different variations of the theme of regulated DUX4 expression in a transgenic mouse (a mouse with the human DUX4 gene inserted in the genome) and all three produce fairly similar results and are currently in use around the world for preclinical testing of FSHD therapeutics.
- Bosnakovski et al, “Muscle pathology from stochastic low level DUX4 expression in an FSHD mouse model” (2017) Nature Communications 8:550, Jones and Jones “A cre-inducible DUX4 transgenic mouse model for investigating facioscapulohumeral muscular dystrophy” (2018) PLoS One 13:e0192657, and Giesige et al., “AAV-mediated follistatin gene therapy improves function outcomes in the TIC-DUX4 mouse model of FSHD” (2018) JCI Insight 3:e123538.
For excellent reviews of FSHD pathogenic mechanisms, see:
- Sidlauskaite et al., “DUX4 Expression in FSHD Muscles: Focus on Its mRNA Regulation” Journal of Personalized Medicine 10:73.
Everyone expresses the DUX4 gene at the 4-cell stage of human development.
At the 2020 annual FSHD International Research Consortium, two groups highlighted the difficulty of an accurate clinical diagnosis of FSHD and the problems with the current state of poor accessibility to FSHD genetic testing. The University of Iowa Molecular Pathology Laboratory, one of the leading FSHD diagnostic labs in the world, reported that only 707 out of 1,594 cases tested for FSHD were in fact genetic FSHD (44.3%). In addition, Dr. Alberto Rosa’s group at IRNASUS-CONICET-UCC in Argentina reported that only 55% of 156 clinical FSHD cases from Latin America were confirmed as FSHD1. Both outcomes show the difficulty in clinical diagnosis of FSHD and the need for better FSHD diagnostics.
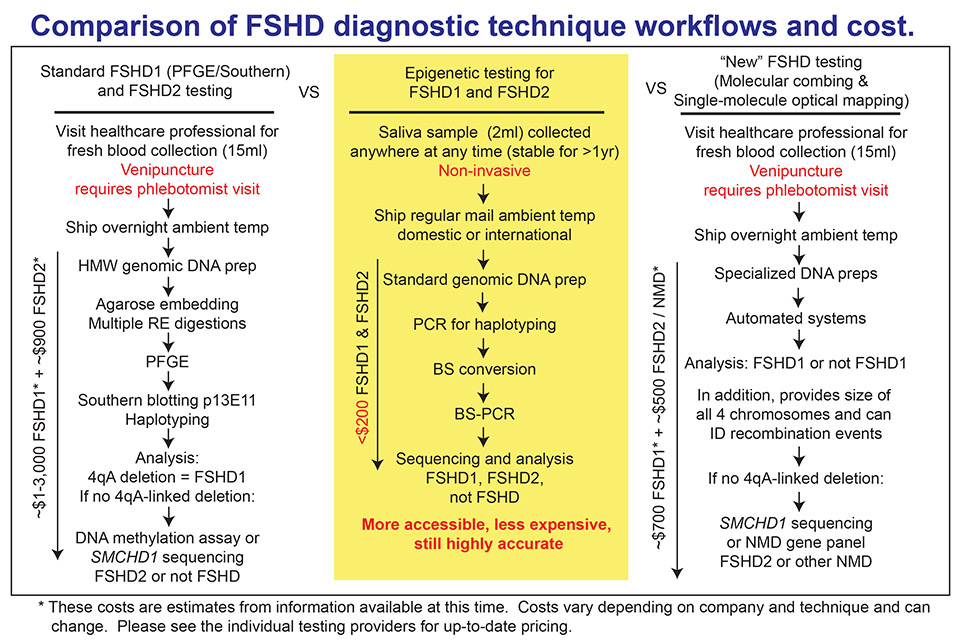
The original genetic diagnostic for FSHD involves isolation of DNA from blood, digestion with multiple sequence-specific DNA cutting enzymes resolution of sizes on a gel and probing for the correct regions with multiple radioactive DNA probes. The results tell you the size of all 4 D4Z4 arrays (two on chromosome 4 and two on chromosome 10) and more recently if they are FSHD-permissive or not. This is still used today; however, more recently, technology has been developed that gives the same information, but is automated and does not require so many manipulations. Both molecular combing and optical mapping (discussed in our Diagnostics for FSHD area) have reduced the time and cost of FSHD testing, but are still limited by the requirement for DNA freshly isolated from blood or cells. In addition, none of these genetic tests will identify FSHD2.
Since FSHD is an epigenetic disease, new FSHD diagnostic testing has been developed that just evaluates the DNA methylation state of the FSHD region and correlates this with FSHD1, FSHD2, or not FSHD. The advantages of this type of testing are that it can be performed on any type of DNA, including DNA obtained from saliva and previously frozen DNA, and it is very quick and low cost. Thus, epigenetic testing removes barriers to FSHD diagnostics around the world. The drawback is that one does not get the size of the D4Z4 array.
MORE ABOUT FSHD DIAGNOSTICS ON OUR FSHD TESTING PAGE
Genetics and the role they play with FSHD
Your genome (your complete set of DNA, including all of its genes) is made up of approximately 6.4 billion base pairs of DNA (about 3.2 billion from Mom and 3.2 billion from Dad). This genome is organized into 23 pairs of chromosomes (#1-22 + X/X or X/Y), and the FSHD region is on chromosome 4q35.2.
WATCH THE FOLLOWING VIDEO FOR A QUICK LOOK AT GENETICS AND FSHD. THEN, TAKE A DEEP DIVE INTO GENETICS AND THE ROLE THEY PLAY WITH FSHD BY DIGGING INTO THE INFORMATION BELOW THE VIDEO.
FSHD genetics
Dive deep ...
Learn about FSHD genetics in the area below. Click the red accordion bars to open/close them.
All forms of FSHD are associated with the chromosome 4 D4Z4 array.
- Model for FSHD1 and FSHD2.
- FSHD1, including infantile (early-onset) is caused by deletions in the D4Z4 array.
- FSHD2 is caused by mutations in other genomic locations.
- Learn about the differences between FSHD1 and FSHD2: English and Español.
FSHD1 is monogenic and has autosomal dominant inheritance. FSHD1 can be asymptomatic and nonpenetrant within families. Asymptomatic individuals are genetically FSHD1 and have no reported muscle weakness but do have signs of FSHD upon clinical evaluation. Nonpenetrant mutation carriers are people who are genetically FSHD1 but do not have signs of muscle weakness upon clinical evaluation.
FSHD2 is digenic (requires two genes) and thus, can seemingly skip generations. Thus, two unaffected (not FSHD2) parents, neither of which meet the genetic requirements for FSHD2, can produce a child that is FSHD2.
All forms of FSHD require a specific type of DNA sequence distal to the chromosome 4 D4Z4 array. The sequence (termed “4A”) is “permissive” for FSHD because it is required to develop FSHD, but in and of itself, it does not cause FSHD.
- FSHD permissive genetics (simplified explanation).
- FSHD permissive vs. nonpermissive (advanced explanation).
Epigenetics refers to the context of your DNA sequence and plays a role in keeping genes on or off.
Your genetics — or your DNA sequence — can be thought of as a wire with the four bases of DNA linearly arranged in specific order of all your genes — your 50,000 plus genes and your 23 pairs of chromosomes.
Epigenetics can be thought of as a different type of wire — more like a coil — because of how your genetics are packaged into your genome. And they’re packaged in such a way that some genes are going to be unaccessible, and other genes are going to be accessible genes that need to be on and expressed.
How does this relate to FSHD? The DUX4 gene typically is off and it is not accessible, and it is repressed. In FSHD, the DUX4 gene context has changed — it is now accessible and it is expressed. And this is mediated by epigenetic marks or epigenetic factors, most common of which is DNA methylation. The DUX4 gene is heavily methylated and it is transcriptionally silent and repressed and off.
In FSHD, it is unmethylated and the gene is actually accessible — is epigenetically on — and the DUX4 gene is expressed now and leads to FSHD. What makes this epigenetic is that these states of being on or off are heritable — that’s a hallmark of epigenetics. The same DNA sequence, different context, heritability. That’s epigenetics in a nutshell.
LEARN MORE BY WATCHING THE EPIGENETICS VIDEOS BELOW AND BY VISITING OUR FSHD TESTING PAGE.
What is epigenetics?
For a quick look at epigenetics, watch this short version of the video.
What is epigenetic research testing?
Preimplantation Genetic Diagnosis (PGD)
FSHD is a genetic disease and thus can be passed on from one generation to the next. If you have FSHD1, there is a 50% chance to pass your FSHD on to each child. Since we now know the genetic causes of FSHD, the technology exists to perform PGD when doing in vitro fertilization (IVF).
We understand that this can be controversial and is definitely not for everyone. We are not advocating one way or another. However, we have found that this is a common question that gets asked, and we just want to make the information available to those who are interested.
For more information, please see the following video by Dr. Ryan Wuebbles, an FSHD patient and researcher who chose this technology for his own family planning.
Preimplantation Genetic Diagnosis (PGD)
FSHD resources from U.S. and global labs, organizations, online communities, more
LABS
Curing FSHD is a worldwide effort. Below are links to websites for a few of the many laboratories around the world working toward finding a cure for FSHD. We apologize for any that we have missed and are happy to add your lab website.
USA
- Peter and Takako Jones Lab
- Robert J. Bloch, PhD, University of Maryland, Baltimore
- Robert K. Bradley, PhD, Fred Hutchinson Cancer Research Center
- Joel Chamberlain, PhD, University of Washington School of Medicine
- Yi-Wen Chen, PhD, MS, DVM, Children’s National Medical Center
- Jason D. Doles, PhD
- Scott Q. Harper, PhD, Nationwide Children’s Hospital
- Sujatha Jagannathan, PhD, University of Colorado Anschutz Medical Campus
- Lou Kunkel, PhD, Harvard Medical School and Boston Children’s Hospital
- Michael Kyba, PhD, Lillehei Heart Institute and University of Minnesota
- Angela Lek, PhD, Yale University
- Jeffrey Boone Miller, PhD
- Ali Mortazavi Lab at UC Irvine
- Fran Sverdrup, PhD, Saint Louis University
- Stephen Tapscott, MD, PhD, Fred Hutchinson Cancer Research Center
- Robert Weiss, PhD, University of Utah
AUSTRALIA
- Marnie Blewitt, PhD, The Walter and Eliza hall Institute of Medical Research, University of Melbourne
- Paul Gregorevic, PhD, The University of Melbourne
- Ian Woodcock, MBBS, BSc, MSc, MRCPCH, FRACP, Murcosh Children’s Research Institute, University of Melbourne
CANADA
LABS (continued)
EUROPE
- Alexandra Belayew, PhD, University of Mons, Belgium
- Frédérique Coppée, PhD, University of Mons, Belgium
- Julie Dumonceaux, PhD, University College London, UK
- Davide Gabellini, PhD, IRCCS San Raffaele Scientific Institute, Italy
- Jessica de Greef, PhD, Leiden University Medical Center, The Netherlands
- Peter Lunt, PhD, Bristol University, UK
- Frederique Magdinier, PhD, Aix Marseille University
- Frédéric Relaix
- Enzo Ricci, MD, Fondazione Policlinico Universitario A. Gemelli IRCCS, Italy
- Giulia Ricci
- Giorgio Tasca, MD, Catholic University, Italy
- Silvere M. van der Maarel, PhD Leiden University Medical Center, The Netherlands
- Yegor Vassetzky, PhD, Lomonosov Moscow State University, Russia and Institut Gustave Roussy, France
- Peter Zammit, PhD, King’s College London, UK
SOUTH AMERICA
FSHD-FOCUSED CENTERS
PATIENT-DRIVEN PROJECTS
ORGANIZATIONS
United States
- Friends of FSH Research
- FSHD Society
- Morgan Hoffman Foundation
- Muscular Dystrophy Association
- The Chris Carrino Foundation for FSHD
International
- ABRAFEU – Associação Brasileira de Facio-Escápulo-Umeral
- AFM-Téléthon
- AMIS FSH
- Fondation DUX
- FSHD Canada Foundation
- FSHD Global Research Foundation Ltd.
- FSHD Spain
- FSHD STICHTING
- Muscular Dystrophy Foundation of South Africa
- Muscular Dystrophy UK
- TREAT-NMD Neuromuscular Network
- Online communities: There are several FSHD groups on Facebook overseen by people with FSHD. Please contact us if you have an interest in learning about connecting with these groups.